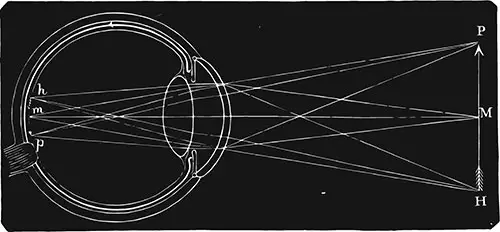
The first FDA-approved eye drop to treat presbyopia is here! Presbyopia is a condition that results in reduced near vision as we age. It occurs because the clear lens behind the iris that once easily changed shape to focus light onto the retina, gets larger and harder over time. This results in a gradual reduction in its ability to focus or what we call in the business “accommodation.”
It has long been the Holy Grail of drug companies to develop a medication to mitigate this affliction that rocks so many of our 40-50-year old’s worlds. The difficulty has always been improving the older patient’s near vision without harming their distance vision, relieving them of the need to reach for reading glasses every time they glance at their cell phone.
AbbVie (who now owns Allergan) submitted an NDA (New Drug Application) to the FDA in February 2021 for its formulation AGN-190584 (Vuity) after it showed considerable promise in all 3 phases of testing. Finally at the end of 2021 we will no longer be reaching for reading glasses but putting a single drop in the eyes during your morning routine.
Vuity is an optimized, essentially diluted, formulation of a well-known ocular medication called pilocarpine. This medication at a higher concentration was previously used to treat glaucoma by shrinking the pupil size and allowing more outflow of fluid, thus lowering eye pressure. The mechanism is the same for presbyopia. However, the smaller pupil in this case, improves what is called “depth of focus.” Ultimately this may improve distance and near vision. Because a smaller pupil will eliminate peripheral distortions of the cornea, it also holds promise for those afflicted with night glare following laser vision correction.
Two main studies, named GEMINI 1 and Gemini 2, consisted of 750 patients who were given a placebo or Vuity. In both studies, Vuity met the primary endpoint reaching statistical significance in improvement in near visual acuity in low light conditions without a loss of distance vision vs. the placebo. There were no serious adverse events observed in any Vuity treated participants. The most common treatment side effects, occurring at a frequency of less than 5%, were headache and conjunctival hyperemia.
While the medication is likely to mitigate the annoyance of reaching for reading glasses just to check a text message, prolonged near work in the face of aging and presbyopia will still require reading correction. However, we are excited for what benefits this medication holds for our patients and it is available now!